UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the
Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): March 20, 2019
SYNLOGIC, INC.
(Exact name of registrant as specified in its charter)
|
Delaware
|
001-37566
|
26-1824804
|
||
|
(State or other jurisdiction
of incorporation)
|
(Commission
File Number)
|
(IRS Employer
Identification No.)
|
|
301 Binney St., Suite 402
Cambridge, MA
|
02142
|
|
|
(Address of principal executive offices)
|
(Zip Code)
|
Registrant’s telephone number, including area code: (617) 401-9975
Not applicable
(Former Name or Former Address, if Changed Since Last Report)
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any
of the following provisions (see General Instruction A.2. below):
☐ Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐ Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐ Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐ Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (17 CFR
§230.405) or Rule 12b-2 of the Securities Exchange Act of 1934 (17 CFR §240.12b-2).
Emerging Growth Company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with
any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☒
Item 7.01. Regulation FD Disclosure.
Synlogic, Inc. (“Synlogic”) has prepared an investor presentation to be used in connection with general corporate presentations. A copy of the
presentation is furnished with this Current Report on Form 8-K as Exhibit 99.1.
The information in Item 7.01 of this Current Report on Form 8-K and Exhibit 99.1 attached hereto is intended to be furnished and shall not be
deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934 (the “Exchange Act”) or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act
of 1933 or the Exchange Act, except as expressly set forth by specific reference in such filing.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its
behalf by the undersigned hereunto duly authorized.
|
|
SYNLOGIC, INC.
|
|
|
|
Date: March 20, 2019
|
|
|
|
|
|
By:
|
/s/ Todd Shegog
|
|
|
|
Name: Todd Shegog
|
|
|
|
|
Title: Chief Financial Officer
|
||
Exhibit 99.1

Synlogic DESIGNED FOR LIFE Corporate PresentationMarch 2019

Forward Looking Statements This presentation contains “forward-looking statements” that involve
substantial risks and uncertainties for purposes of the safe harbor provided by the Private Securities Litigation Reform Act of 1995. All statements, other than statements of historical facts, included in this presentation regarding strategy,
future operations, future financial position, future revenue, projected expenses, prospects, plans and objectives of management are forward-looking statements. In addition, when or if used in this presentation, the words “may,” “could,”
“should,” “anticipate,” “believe,” “estimate,” “expect,” “intend,” “plan,” “predict” and similar expressions and their variants may identify forward-looking statements. Examples of forward-looking statements include, but are not limited to, the
approach we are taking to discover and develop novel therapeutics using synthetic biology; statements regarding the potential of our platform to develop therapeutics to address a wide range of diseases, including: inborn errors of metabolism,
liver disease, inflammatory and immune disorders, and cancer; the future clinical development of Synthetic Biotic medicines; the potential of our technology to treat hyperammonemia and phenylketonuria; the expected timing of our anticipated
clinical trial initiations; the benefit of orphan drug and fast track status; the adequacy of our capital to support our future operations and our ability to successfully initiate and complete clinical trials; the results of our collaborations;
and the difficulty in predicting the time and cost of development of our product candidates. Actual results could differ materially from those contained in any forward-looking statement as a result of various factors, including, without
limitation: the uncertainties inherent in the preclinical development process; our ability to protect our intellectual property rights; and legislative, regulatory, political and economic developments, as well as those risks identified under
the heading “Risk Factors” in our filings with the SEC. The foregoing review of important factors that could cause actual events to differ from expectations should not be construed as exhaustive and should be read in conjunction with statements
that are included herein and elsewhere, including the risk factors included in our Annual Report on Form 10-K filed with the SEC on March 12, 2019. The forward-looking statements contained in this presentation reflect our current views with
respect to future events. We anticipate that subsequent events and developments will cause our views to change. However, while we may elect to update these forward-looking statements in the future, we specifically disclaim any obligation to do
so. These forward-looking statements should not be relied upon as representing our view as of any date subsequent to the date hereof.

Synthetic BioticTM Medicines Designing for LIFE Patient NeedThere remain many indications for which
conventional medicines do not provide effective solutions for all patients Conventional Approaches LimitedSingle mechanism agents do not address complex biology, often lead to systemic exposure without control An Engineered Living Medicine
SolutionSynlogic is harnessing nature and technology to create LIVING medicines designed to significantly improve patients’ LIVES

v Synthetic BioticTM Medicines A Novel Class of Engineered Living Medicines Designed
genetic circuits to execute biological functionsDegradation of disease-causing metabolitesProduction of therapeutic molecules Bacterial chassisNon-pathogenicAmenable to genetic manipulation PATHWAYS, COMBINATIONS, BIOMARKERS PROGRAMMABLE
POTENCY AND CONTROL LOCAL ACTIVITY, REDUCED SYSTEMIC TOXICITY BIOTIC SYNTHETIC

Synthetic Biotic Portfolio: Breadth and Potential Initial Applications Designed to Target Different
Sites of Action in Metabolic and Immunomodulatory Diseases Oral Administration IMMUNOMODULATION METABOLIC DISEASES Immuno-Oncology Inflammatory and Autoimmune Small or Large Intestine “Cold” Solid Tumors Small or Large Intestine Rare
MetabolicDisease BroadMetabolicDisease

Synthetic Biotic Portfolio Hyperammonemia – Urea Cycle Disorder Research IND-Enabling
Studies Phase 1 Phase 2 Phenylketonuria Additional Rare Metabolic Diseases Hyperammonemia – Hepatic Encephalopathy (HE) Inflammatory Bowel Disease Immuno-Oncology Solid Tumors Additional Oncology
Applications SYNB1020 SYNB1618 SYNB1020 SYNB1891 Rare Metabolic DiseasesBroad Metabolic DiseaseImmunomodulation

UREA CYCLE DISORDERS (UCD) SYNB1020 for Hyperammonemia Indications Characterized by Systemic Ammonia
Accumulation Neuropsychiatric complication in patients with end-stage liver disease (cirrhosis)Liver dysfunction leads to ammonia accumulationToxic to brain, leading to HE crisis & hospitalizationPatients: 165,000 diagnosed overt patients
in USUp to 70% of patients with cirrhosis characterized as covert (subclinical)Treatment: Lactulose: laxative with significant side effectsRifaximin: reduction in overt HE recurrence Genetic defects in Urea CycleDeficiency in one of the six
enzymes Nitrogen accumulates as toxic ammonia leading to metabolic crisisPatients: ~2,000 diagnosed in US; similar in EUTreatment: Ammonia scavengers: Buphenyl® (sodium phenylbutyrate), Ravicti® (glycerol phenylbuterate)Low protein diet with
amino acid supplements Target Profile to Address Unmet Need:Reduce episodes of hospitalizationImprove cognitive outcomes, Quality of Life Target Profile to Address Unmet Need:Maintain blood ammonia in normal range, avoid crisisProtein
liberalization: 50-100% more per dayOral administration HEPATIC ENCEPHALOPATHY (HE)

Arginine Urea SYNB1020 Mechanism of Action: Under normal conditions, the urea cycle metabolizes
ammonia into ureaIn UCD and HE, ammonia is not efficiently metabolized via urea cycle. SYNB1020 provides an alternative mechanism Conversion of Toxic Ammonia into Beneficial Arginine for the Treatment of UCD and
HE Ammonia/NH4Cl UreaCycle Ammonia Arginine Arginine argD argF argI argG carA carB argR argE argC argB argH Metabolic Conversions FNR FNR argAfbr Glutamate Converts ammonia to Arginine Engineered Probiotic
Bacteria: E. coli NissleComponents of Synthetic Genetic Circuit Can re-enter the Urea Cycle Ammonia metabolism blocked in disease

In vivo data in mouse models and healthy volunteers demonstrate mechanism of action SYNB1020 data
recently published in Science Translational Medicine PLASMA NITRATE URINARY NITRATE MOUSE MODEL

SYNB1020 Clinical Data in Healthy Volunteers Dose-dependent Increase in SYNB1020 in Feces, Clearance
on Cessation of Dosing DOSE-DEPENDENT INCREASE IN FECES CLEARANCE Dosing period = 14 days Dosing period = 14 daysSamples collected daily

SYNB1020 Clinical Development Hepatic Encephalopathy Phase 1b/2a in Patients with Cirrhosis and
Elevated Ammonia Hepatic Encephalopathy Clinical TrialRandomized, double-blind placebo-controlled study ongoing at multiple sites in the US Primary outcome: establish safety/tolerability in patients with cirrhosis and elevated ammoniaSecondary
outcome: reduction of ammonia 2018 2019 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Phase 1b / 2a PROGRAM Hepatic Encephalopathy Mild Cirrhosis Patients, MELD1<12, Open label, TID for 6 days Sentinel cohort for safetyN = 6 Cirrhosis Patients:
MELD < 20 & Elevated Ammonia; RCT, TID for 6 days Proof of MechanismN up to 40 Safety Evaluation Part 1 Part 2 1. MELD score: scoring system model for end-stage liver disease Urea Cycle Disorders(Plans to continue development in
UCD dependent on data from Ph 1b/2a HE study)

PKU is a rare inherited amino acid metabolism disorder Causes build up of amino acid phenylalanine
(Phe) in the bodyToday, less than half of adults are at or below target Phe levels of 120-360 mmol / LIf left untreated, symptoms include cognitive impairment, convulsions, behavioral problems, skin rashPatients:16,500 diagnosed in US, similar
in EU5Treatment: Phenylalanine is found in all proteins therefore low protein diet is followed (no meat, dairy, nuts, eggs)KUVAN® (sapropterin dihydrochloride): PAH cofactor. 20-40% of patients are respondersPalynziqTM (pegvaliase-pqpz):
injectable, pegylated, bacterial enzyme (phenylalanine ammonia-lyase or PAL) for treatment of adult patients SYNB1618 for Phenylketonuria (PKU) Target Profile to Address Unmet Need:Manage Phe below target levels to prevent irreversible
cognitive damageIncrease natural protein intake: classic PKU patients’ natural protein intake is typically less than 10g Oral dosing without systemic toxicity Goal: Managing Plasma Phe Levels

SYNB1618 Mechanism of Action Amino acids from dietary proteins (absorption and
recirculation) Phe PKU Healthy Phenylalanine Hydroxylase (PAH) converts Phe into Tyrosine Tyrosine Accumulation of Phe to toxic levels Impaired PAH SYNB1618 Manage Phe levels When Phe
is not efficiently metabolized (PKU) SYNB1618 provides an alternative mechanismPAL3: produces TCA which is converted to HA in the liver and is excreted in urineLAAD: produces phenylpyruvate (PP) Phenylalanine (Phe) Hippuric Acid
(HA) Engineered Probiotic Bacteria: E. coli NissleComponents of Synthetic Genetic Circuit trans-Cinnamic Acid (TCA) PheP: High-Affinity Uptake trans-Cinnamic Acid (TCA) pheP PAL3 Metabolic
Conversions FNR FNR FNR FNR Phenylalanine LAAD AraC AraC Phenylalanine (Phe) Phenylpyruvate (PP)

Iteration Potential Advantage of Synthetic Biotic Design Build Test
Cycle With pheP Transporter Without pheP Transporter 20 10 10 0 5 Phe Degradation
Rate(μmoles/h/10e9cells) IntegrationSite PAL copyNumber IntegrationSite PAL copyNumber pheP copyNumber LAAD +3XPAL + pheP+ SYNB1618 LEAD OPTIMIZATION IN VITRO COLLABORATION

SYNB1618 Preclinical Characterization Biomarkers Demonstrate Activity of SYNB1618 in Mouse Model of
PKU and Healthy NHPs IN VIVO EFFICACY IN (PKU) PAHenu2/enu2 MOUSE DOSE RESPONSE IN HEALTHY NHP’s Nat. Biotechnol. 2018 Oct;36(9):857-864 Development of synthetic live bacterial therapeutic for the human metabolic disease
phenylketonuriaVincent M Isabella et al, Synlogic, Inc.

SYNB1618 in the Clinic: Safety There were no treatment-related serious adverse events, no systemic
toxicity or infections Treatment-emergent adverse events were either mild or moderate in severity, and reversible. Most adverse events were GI-relatedSingle dose MTD was defined as 2x1011 CFU. Doses above this level were associated with
dose-limiting GI adverse eventsAll subjects cleared the bacteria. There was no evidence of colonization, and no subject required antibiotics Interim Analysis of Phase 1/2a SAD/MAD Study Demonstrates Safety and Clearance in Healthy
Volunteers Based on pharmacodynamic data and tolerability profile, a dose of 7x1010 CFU was identified for the second part of the study in PKU patients 56 healthy volunteers Received at least one dose of SYNB1618 or placebo AdultsAge range:
18-62 yrs old

SYNB1618 in the Clinic: Activity Statistically Significant Dose-dependent Activity of SYNB1618 in
Healthy Volunteers SYNB1618 or placebo Protein shake /meal D5-Phe Measure over 6hrs:Plasma:Phe/D5-PheTCA/D5-TCAUrine: HA/D5-HA TCA AUC SINGLE DOSE RESPONSE MAD URINARY HA AND D5-HA Key: HA: Hippurate, D5-HA: labeled HA, CFB:
change from baseline, CFP: change from placebo

SYNB1618 Clinical Development Phase 1/2a in Healthy Volunteers with Patient
Cohort 2018 2019 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Phase 1 / 2a Phase 1 / 2a SAD / MAD Healthy Volunteers SD / MDPKU Patients PROGRAM PKU Clinical Trial DesignRandomized, double-blind placebo-controlled study ongoing at multiple sites
in the US Primary outcome: establish safety/tolerability following single and multiple doses in HV and PKU patients Secondary outcome: SYNB1618 kinetics in fecesExploratory: change from baseline in plasma and urinary biomarkers Single
Ascending Dose (SAD)Healthy Volunteers6 cohorts, N = 24 Multiple Ascending Dose (MAD)7 daysHealthy Volunteers; 4 cohorts, N = 32 MTD Dose ID Single Dose (SD)PKU PatientsN = 4 Multiple Dose (MD)7 daysPKU Patients; N up to 20 Dose
Confirm

© 2019 SYNLOGIC. ALL RIGHTS RESERVED. | 19 Immuno-Oncology

CHECKPOINT INHIBITORS HAVE TREATMENT FAILURES Synlogic Vision for Immuno-Oncology Expand the
Benefits of Immunotherapy Broadly Across Tumor Types Other tumors, where CPIs are not indicated, show little-to-no response to checkpoint inhibitors Failure Rates for Select FDA Approved CPI Monotherapy 55% NSCL 1st
line 60% Melanoma 1st line 71% Bladder 1st line 87% Cervical / Gastric 2nd line Non-responders For indications where immune checkpoint inhibitors are indicated, 55-87% of patients fail to respond Nature often gives us
hints to her profoundest secrets, and it is possible that she has given us a hint in which, if we will but follow, may lead us on to the solution of this difficult problem. “ ” Bacteria Recognized as Earliest Immunotherapy DR. WILLIAM B.
COLEYIMMUNO-ONCOLOGY PIONEER Enable broad response and remission through engagement of multiple immunomodulatory pathways to enhance tumor inflammation and promote robust T cell responses

A Tumor Can Evade Multiple Critical Aspects of the Cancer-Immunity
Cycle Killing Recognition Infiltration Antigen release Presentation Priming and activation T cell trafficking Insufficientactivity/proliferation Immuno-suppression Insufficient trafficking Insufficient priming Recognized Need
to Combine Mechanisms to Broaden the Benefit of Immunotherapy Adapted from Chen, Melman; Immunity 2013 MONOTHERAPIES OFTEN FAIL TO OVERCOME TUMOR EVASION MECHANISMS Rationally Designed for Combinatorial EffectLocally Inflame the tumor
microenvironment (TME)Systemically Drive Tumor-Antigen Specific Immunity In Situ Vaccination: Neo-antigen Priming and Sustained Immune Response ENGINEER LIVING SOLUTIONS: SYNTHETIC BIOTIC MEDICINES

BEHAVIOR WITHIN TUMOR CHASSIS DISTRIBUTION Intra-tumoral Injection of Synthetic Biotic Chassis: Tumor
Colonization Without Leakage; Local Innate Immunity Robust proliferation in tumor.No significant leakage in B16.F10 Mice Survival/proliferation in tumors 10-15 days post-single dose. Potential for limited injections Elicits innate
responses (IL-6 and TNFα) in the tumor. Not in circulation 30 mins 24 hrs 72 hrs Image of Tissue Reporter Signal TumorCross Section

Dual Innate Immune Activator:Synthetic Biotic Medicine Producing STING Agonist (SYNB1891) Synthetic
biology applied to confer activities for efficacy and control for safetyDesigned as a dual innate immune activator: combined benefit of bacterial chassis and STING agonistThe dacA gene is integrated into genome under the control of inducible
promoter (Pfnr) to produce c-di-AMP (CDA)Dual biosafety feature via auxotrophies – no proliferation in tumor, systemic circulation or environmentLearnings inform future combinations ANAEROBIC ENVIRONMENT dacA Pfnr ATP
+ATP Cyclic-di-AMP(STING Agonist) 02 AuxotrophiesDiaminopimelic acid (DAP)Thymidine

Innate Immune Activation through Multiple Pathways Uniquely Signals Through CDN-STING and Bacterial
Chassis in Target Cells to Drive Efficacy CDN-STINGActivation SYN-STING Naked STING Agonist Gram-negative BacteriaE. coli Nissle TLR4 IFN-b1 Type 1 IFN P IRF3 Phagosome CDNsC-di-AMP STING BACTERIALTLR/MyD88
Signaling TNF, others CYTOSOL NUCLEUS Gram-negative BacteriaE. coli Nissle TLR4 IL-6, p50 p65 NF-κB APC BACTERIALIntracellular TLR4 Signaling TLR4 Gram-negative BacteriaE. coli Nissle IFN-b1 Type 1
IFN P IRF3 Phagosome TRIF TRAM BACTERIALcGAS-STING Activation Gram-negative BacteriaE. coli Nissle TLR4 IFN-b1 Type 1 IFN P IRF3 2’3’-cGAMP STING cGAS dsDNA(pathogen,
host) Promotes Trafficking, Immune Activation/Proliferation, Priming APC APC APC TUMOR

SYNB1891 In Vitro Characterization Interferon Production Across Multiple Human STING Alleles Greater
than Naked STING Agonist Additional Proinflammatory Pathways Engaged HUMAN PRIMARY DENDRITIC CELLS REPORTER HUMAN MONOCYTIC LINE Human STING Alleles STING Knockout
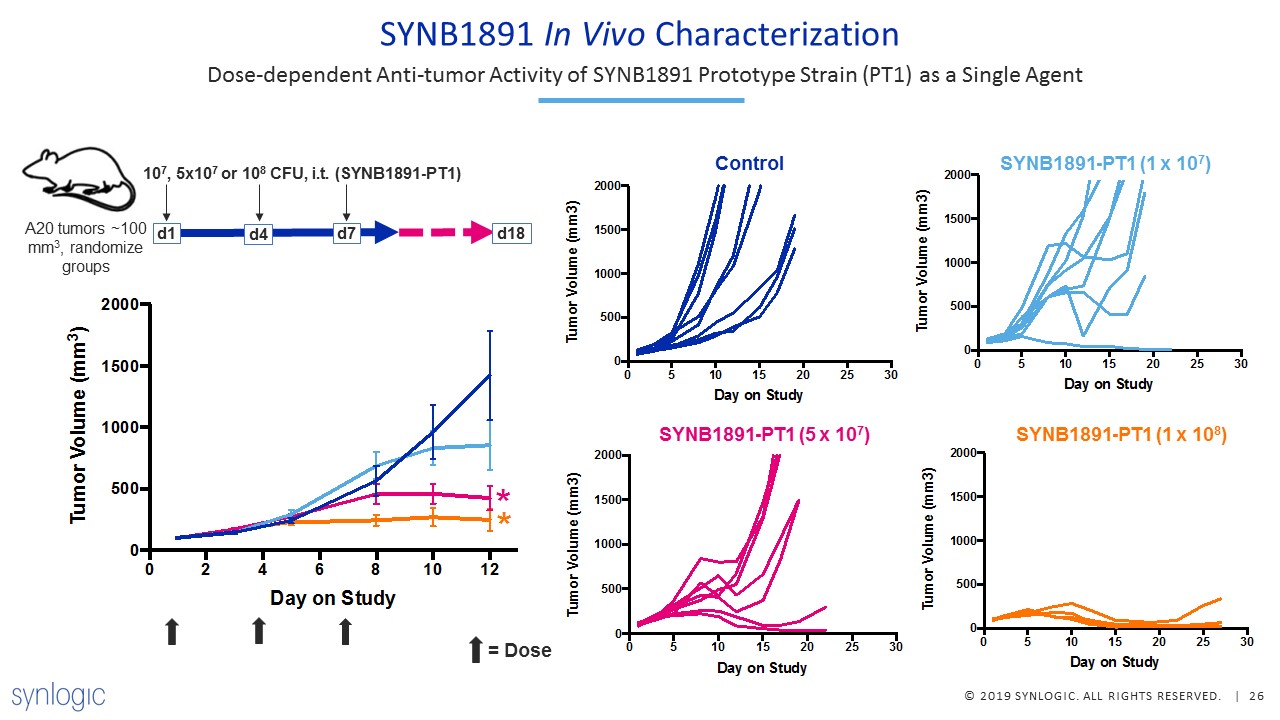
SYNB1891 In Vivo Characterization d1 107, 5x107 or 108 CFU, i.t. (SYNB1891-PT1) A20 tumors ~100 mm3,
randomize groups d7 d4 d18 Dose-dependent Anti-tumor Activity of SYNB1891 Prototype Strain (PT1) as a Single Agent Control Control SYNB1891-PT1 (1 x 107) SYNB1891-PT1 (5 x 107) SYNB1891-PT1 (1 x 108) = Dose

SYNB1891 In Vivo Characterization SYNB1891 Prototype Strain (PT1) Leads to Systemic Anti-tumor
Immunity SYNB1891-PT1 dose Re-challenge d1 108 CFU, i.t. (Bacteria) A20 tumors ~80-100 mm3, randomize groups d7 d4 d35 d63 Rechallenge (2e5 A20 cells) d1
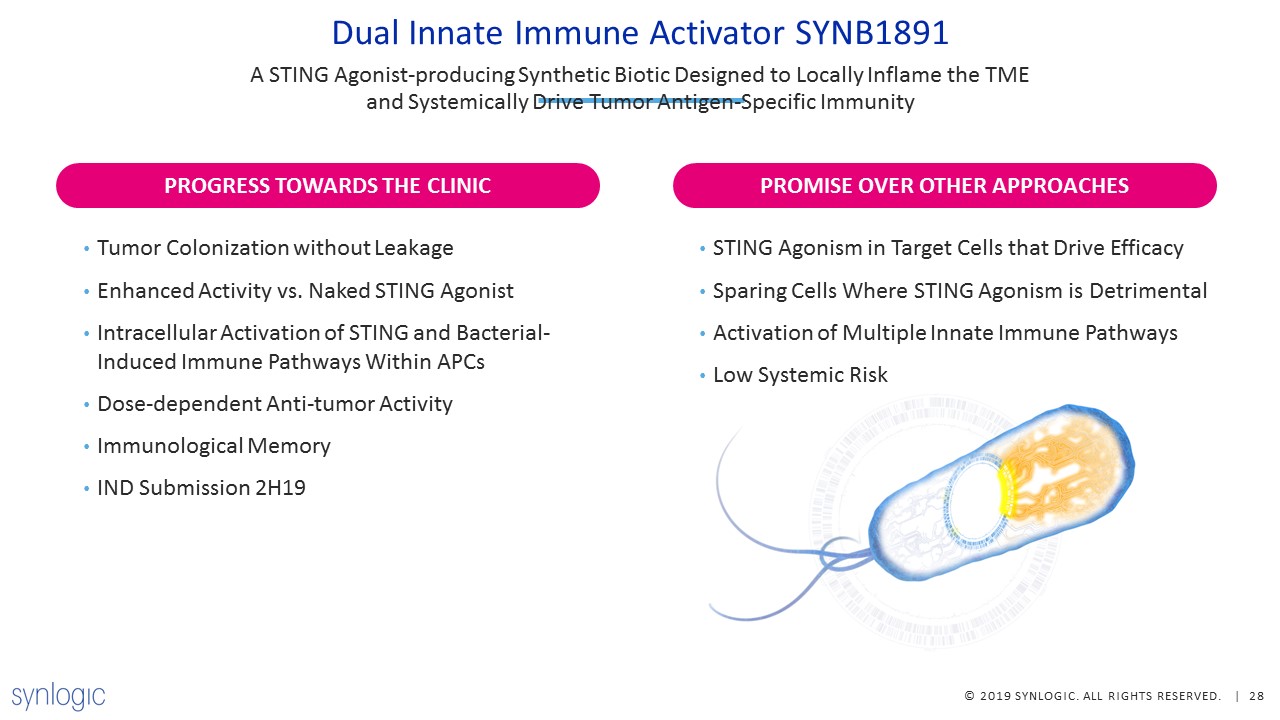
Dual Innate Immune Activator SYNB1891 A STING Agonist-producing Synthetic Biotic Designed to Locally
Inflame the TME and Systemically Drive Tumor Antigen-Specific Immunity Tumor Colonization without LeakageEnhanced Activity vs. Naked STING AgonistIntracellular Activation of STING and Bacterial-Induced Immune Pathways Within APCsDose-dependent
Anti-tumor ActivityImmunological MemoryIND Submission 2H19 STING Agonism in Target Cells that Drive EfficacySparing Cells Where STING Agonism is DetrimentalActivation of Multiple Innate Immune PathwaysLow Systemic Risk PROMISE OVER OTHER
APPROACHES PROGRESS TOWARDS THE CLINIC

Additional Synthetic Biotic Effectors VISION: Rational Design to Locally Inflame the TME AND
Systemically Drive Tumor-Antigen Specific Immunity Chassis effectCXCL10Hyaluronidase Kyn ConsumptionAde ConsumptionαPD-1 scFv Chassis effect5FC5FUSTINGαCD40 scFv/CD40L TNFαIFNγαCD47 ScFv / SirpαGM-CSF TUMOR LYMPH NODE Systemic
Tumor-Antigen Specific Immunity Locally Inflame the TME IL-15; IL-12Arg Production4-1BBLOX40L RELIEVE IMMUNOSUPRESSION PROMOTE AND SUSTAIN IMMUNE ACTIVATION PRIME FOR TUMOR-ANTIGEN-SPECIFIC VACCINATION PROMOTE TRAFFICKING

Broad Ambitions in Immuno-Oncology SYNB1891 DISCOVERY PORTFOLIO INTRATUMORAL Vision: Expand
and Exceed the Effect of Cancer Immunotherapies COMBINATIONS HARNESS THE MICROBIOME ORAL

Synlogic Internal GMP Manufacturing Capabilities In-house Process Development and Clinical
Manufacturing for Early & Mid-Stage Trials Biotherapeutic Manufacturing Candidate Selection & Process Dev. Testing (in vitro and in vivo) Strain Engineering OptimizedProcess Scale-up Process development and lab
scale production MicrobioreactorsHigh throughput strain screening & process development Fermentation DownstreamHarvest/Formulation Lyophilization, milling, & capsule fill Discovery Lead Optimization Pre-IND Phase 1 Mid-Stage
Trials Analytical Methods Development and Validation

Progress and 2019 Milestones 2019 Milestones SYNB1618 in PKUComplete ongoing study in patientsData
expected mid-2019 (safety, tolerability and biomarkers)SYNB1020 in HyperammonemiaPreclin. and HV clin. data published in Sci. Transl. Med.Complete ongoing study in patients with cirrhosisData expected mid-2019 (safety, tolerability and
ammonia-lowering)With ammonia-lowering data define development planSYNB1891 in Immuno-OncologyIND submission 2H2019Advance AbbVie collaboration Advance preclinical pipeline 2018 Accomplishments SYNB1618 in PKUPreclinical data published in
Nature BiotechnologySafe, well-tolerated, proof of mechanism in HVsFDA Fast Track DesignationSYNB1618 and SYNB1020 Initiated studies in patients Established in-house manufacturing capability for mid-stage clinical studies. Developed path to
solid oral formulationIO Lead Candidate, SYNB1891, selected Initiated IND enabling studiesAdvanced AbbVie collaboration

© 2019 SYNLOGIC. ALL RIGHTS RESERVED. | 33 Synlogic is designing microbes that are engineered to
compensate for missing functions in a variety of diseases The Company has demonstrated that Synthetic Biotic medicines function as designed in humans Synlogic is building a path to a broad portfolio of products that could change patients’
LIVES

301 BINNEY ST., #402, CAMBRIDGE, MA 02142TEL: 617-401-9975WEB: WWW.SYNLOGICTX.COM | EMAIL:
INFO@SYNLOGICTX.COM© SYNLOGIC. CONFIDENTIAL. ALL RIGHTS RESERVED.